The branch of chemistry that deals with the study of those compounds that contain carbon (C) and hydrogen(H) called hydrocarbon and their derivatives except oxide of carbon, carbonates, bicarbonates, cyanide, etc. are called organic chemistry.
Organic Compound:- These compounds mostly contain carbon and hydrogen and in a few cases contain other elements such as Oxygen, Nitrogen, Sulfur, Phosphorous, and Halogens.
{tocify} $title={Cover in Topic}
Types of Organic Compounds
There are two types of organic compounds based on their origin. (1)- Naturally occurring organic compounds e.g. Coal, Food, Petrol, Natural gas, etc. (2)- Synthetic organic compounds e.g. Plastic, Detergents, Medicine, etc.
Inorganic Compounds:- These compounds that contain their derivatives are called inorganic compounds. For Exam. HCL, H2SO4, Sodium Chloride, Carbon dioxide, CaCO3, NaHCO3 etc. These are some compounds that contain carbon but they are inorganic i.e. CO, CO2, CaCO3.
Representation of Organic Compound
Organic compound representation in the following ways. (i) Molecular formula (ii) Structural formula (iii) Condensed formula (iv) Dot and cross formula.
(i) Molecular Formula:- The formula that shows the actual number of atoms of different elements present in one molecule of organic compound is called molecular formula.
Example: The molecular formula of benzene is C6H6. That benzene has six carbon atoms and six hydrogen atoms.
(ii) Structural Formula:- The arrangement of atoms of different elements around the carbon atoms present in a molecule of organic compound is called structural formula.
Characteristics of Structural Formula
It shows the number and types of atoms present in a molecule and also shows the bonding arrangement of atoms. (a) In the structural formula, all the bonds are shown with their exact number. (b) A single bond is represented by a single line ( 一 ) between atoms. (c) The double bond is represented by a double line ( 二 ) and a triple bond is represented by a triple line ( ☰ ) between bonded atoms.
Example:- The structural formula of propane and butane are given below.
(iii) Condensed Formula:- The formula where the group of atoms are shown in the order as they appear with the structural formula with no bond or dashes is called condensed formula. Example: Hexane contains six carbons and fourteen hydrogen, and its condensed formula is CH3(CH2)4CH3.
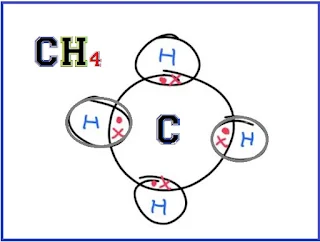
(iv) Dot and Cross Formula:- The structural formula in which electrons are shown as dos and crosses between various atoms in one molecule of an organic compound is called the dots and cross formula. Example: The dots and cross formula for methane are given below in which the electrons of carbon are represented by dot ( • ) and the electrons of hydrogen are represented by the cross ( ✖ ).
Classification of Organic Compound
Organic Compounds are mainly classified into two types based on their carbon skeleton. (i) Open chain or acrylic organic compounds. (ii) Closed chain or cyclic organic compounds.
(i) Open Chain Organic Compound
The organic compound in which carbon atoms are in open-chain structures is called open chain or acyclic organic compound. They are further classified into straight-chain and branched-chain organic compounds.
(a) Straight Chain Organic Compound
the organic compounds that contain the straight chain of carbon atoms in their molecule are called straight-chain organic compounds their common names have a prefix (n) with corresponding alkanes.
Exp.
N-butane ( CH3一CH2ーCH2一CH3 ) and n-pentane ( CH3-CH2-CH2-CH2-CH3 ).
(b) Branched Chain Organic Compound
The organic compounds in which all carbon atoms are not arranged in a linear sequence but have alkyl groups attached to them and form branch structures are called branched-chain organic compounds. The name has the prefix ISO with corresponding alkanes.
(ii) Closed Chain or Cyclic Compound
The organic compound in which carbon atoms are linked together to form a closed or ring structure is called a closed chain or cyclic organic compound, they are further divided into two groups. Homocyclic organic compounds and heterocyclic organic compounds.
(a) Homocyclic Organic Compounds
The organic compound that is in a closed chain or structure and the Ving consists of only carbon atoms is called a homocyclic organic compound. It is further divided into two types. Ali-cyclic and Aromatic Organic compounds.
• Ali-cyclic organic compounds:- The organic compound which is in a closed chain structure forming a ving are called ali-chain cyclic compounds. These organic compounds resemble properties of open-chain organic chain organic compounds but differ in their structure and formulae.
Examples:-
(b) Aromatic Organic Compound
The cyclic organic compounds having alternate single and double bonds in their structure or carbon ving are known as aromatic organic compounds.
Example:- The important members of this are benzene and other organic compounds that are derived from benzene like naphthalene, anthracene, etc.
Heterocyclic Organic Compounds:- The organic compounds that contain one or more atoms than carbon atoms other than carbon atoms such as Sulphur oxygen, and nitrogen in the ring are called heterocyclic organic compounds.
Classification of Organic Compounds based on Bonds
Organic compounds are classified into two types based on the chemical bonds present in them (i) Saturated organic compounds and (ii) Unsaturated organic compounds.
1. Saturated Organic Compounds
Those organic compounds in which all the carbon atoms are bonded to each other through a single covalent bond are called saturated organic compounds. These compounds are also known as alkanes. Example: Methane (CH4), Ethane(C2H6), Propane(C3H3), Butane(C4H8), Pantone(C4H10), etc.
2. Unsaturated Organic Compounds
Those organic compounds that contain at least one double or triple covalent bond between carbon-carbon atoms are called unsaturated organic compounds. Alkenes and Alkynes are unsaturated organic compounds.
Alkenes:- The hydrocarbon which contains at least one double covalent bond between carbon-carbon atoms is called alkenes.
Alkynes:- The hydrocarbon that contains at least one triple bond between carbon-carbon atoms is called alkynes.
Diversity and magnitude of organic compound
The diversity and magnitude of organic compounds are due to the uniqueness of carbon bonding and structure, carbon can form millions of organic compounds due to the following reasons.
(i) Tera-valent:- carbon is a tera-valent atom because a carbon atom can form a total of four covalent bond
(ii) Mode of Bonding:- The carbon atom can form a single double or triple covalent bond in its compound.
(iii) Catenation:- The self-linkage ability of carbon atoms is called catenation carbon atoms can covalently bond with other carbon atoms to form a straight chain branched chain and ving.
(iv) Carbon Bonding to Other Elements:- Besides catenation, carbon atoms can also form bonds with other elements. O2, N2, S halogen etc.
(v) Arrangement of Atoms:- Different arrangement of carbon atoms from different compounds. Example: The molecular formula of ethanol and dimethyl ether are the same (C2H6O) but due to different arrangements of carbon atoms from different compounds.
(vi) Strength of covalent bond of carbon atoms:- The size of carbon atom is very small due to which carbon atoms from the strong covalent bond with each other and with other elements, such as hydrogen, oxygen, nitrogen, and halogen to form a large number of organic compound.
Characteristics of Organic Compounds
Some of the important characteristics of organic compounds are given below.
(a) Composition of Organic Compound:- Organic compounds are mainly composed of carbon, hydrogen, and atoms, they also contain oxygen, Sulphur, nitrogen, and halogen along with carbon and hydrogen atoms.
(b) Covalent Nature of Organic Compound:- The organic compounds are covalently bonded compounds in nature they are mainly non-polar with few exceptions.
(c) Solubility of Organic Compound:- Most organic compounds are non-polar and soluble in non-polar solvents like acetone, and ether.
Some Important Compounds of Copper | Copper Compounds and Uses
Gas Law Equations | State of Matter gases | Properties of Gases
Polar and Non-Polar Bonds: Explanation
Polar covalent bond
The covalent bond which is formed by the mutual sharing of electrons between atoms, having different electronegativities is called polar covalent bond.
Explanation:- When a covalent bond is formed between two dissimilar atoms, the electronegativity of one atom is higher than the other so it attracts electrons more forward itself and forwards a partial negative charge on it while another atom forms a partial positive charge on it, due to these opposite poles are created and polar bond is forward.
Polar bond formation in HCL
In the HCl molecule the Cl is H atoms, there for chlorine has the tendency to attract the shared paired of electrons toward itself by a greater force, so chlorine forms a partial negative charge on itself, while Hydrogen atom forms a partial positive charge on itself, so opposite poles are formed in H-Cl molecule and the bond is said to be a polar covalent bond, Other examples of a polar molecule are. H2O, NH3, etc.
Non-Polar Covalent Bond
The covalent bond which is formed? between atoms having the same electronegativity is called a non-polar covalent bond.
Explanation: A non-polar covalent bond is formed between two similar atoms in, there for both the atoms attract the shared paired electrons equally due to the same electronegativity of toms in non-polar molecules the opposite poles are not created in these bonds. Example: H,2 N2, O2, Cl2, Br2, N2, etc
Hydrogen Bonding
The force of attraction between the positively charged hydrogen atoms of one molecule and a lone pair of electrons of O, N, or F of the other molecule is called hydrogen bonding. A hydrogen bond is an attractive force between a highly electron-deficient species Hydrogen atom and a nearby highly electron-negative atom with a lone pair of electrons such as F, O, or N.
Explanation:- Hydrogen bonding is found in all polar molecules which contain N-H, O-H, and F-H bonds such as H2O, NH3, HF, etc. When hydrogen is contently bonded to high E.N atoms such as O, N, and F, a strong positive charge develops on hydrogen atoms and a negative charge appears on high electron negative atoms when these molecules are close to each other the positive hydrogen of one molecule attracts the high electronegative atom of other molecules as a result strong force of attraction is created.
Properties of Hydrogen Bonding
Hydrogen bonds are stronger than dipole-dipole interaction but weaker than covalent bonds.
It is about 20 times weaker than a covalent bond and 10 times stronger than a dipole-dipole interaction.
Hydrogen bonds form long chains and help in the formation of a network of molecules.
Hydrogen bond is directional.
Application of Hydrogen Bonding
Hydrogen bonding is extremely important in determining the properties of water, and biological molecules such as protein, DNA, etc. The adhesive action of paints and dyes is developed due to hydrogen bonding. Synthetic resins also bind two surfaces.
>Surface Chemistry Notes-Definition, Absorption, Adsorption, Application and Uses