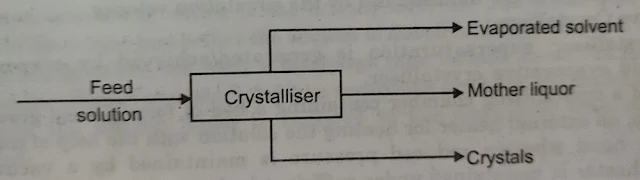
Material balance is used to calculate the yield of the crystallization operation which is the mass of crystals obtained from a given mass of solution. Energy balance calculations in a crystallization process are essential to determine the cooling requirements or final conditions.
{tocify} $title={Table of Contents}
The Overall Material Balance
The mass flow rate of feed solution in kg/h = The kg/h of crystals obtained + The kg/h of mother liquor leaving the crystallizer + The mass flow rate of evaporated solvent in kg/h.
Material balance of solvent
Solvent in feed = Solvent evaporated + Solvent in mother liquor + Solvent in hydrated crystals.
Material balance of solute
Solute in feed = Solute in product crystals + Solute in the mother liquor.
The Energy Balance of the Crystallizer
Energy balance calculations in a crystallizer process are essential to determine the cooling requirements or the final condition. In this calculation heat of crystallization is important and it is the latent heat that evolved when a solid is formed from a solution.
Ordinarily, the crystallization process is exothermic and the heat of crystallization varies with temperature and concentration. Heats of crystallization are not available, but the heat of solution data are available. The process of crystallization is the reverse sign is taken as the heat of crystallization. In case of a cooling crystallization with no evaporation, the heat balance is
Heat to be removed = Q= FCpf ∆T + Cλc
When specific heat data are available for the initial feed solution over a range of temperatures then the heat to be removed is equal to the heat to be removed to cool the feed from the initial temperature (T1) to the final temperature (T2 such that T1>T2) without any crystal precipitating plus the heat liberated due to formation of crystals from the supersaturated solution at the final temperature.
Heat with a solution at T1 + Heat liberated due to crystallization = Heat with a solution at T2 + Heat to be removed.
Where ∆T = cooling range
Cpf = specified heat of feed solution in KJ/kg
F = feed or feed rate
C = crystals formed
λc = heat of crystallization
Take these Notes is, Orginal Sources: Unit Operations-II, KA Gavhane