Heat transfer deals with the study of rates at which the exchange of heat takes place between a hot source and a cold receiver. In process industries, many operations involve the transfer of energy in the form of heat, e.g. evaporation, distillation, drying, etc., and also chemical reactions carried out on a commercial scale take place with the evolution or absorption of heat.
It is also necessary to prevent the loss of heat at the desired rate. The knowledge of laws of heat transfer, mechanisms of heat transfer, and process heat transfer equipment is of great importance from the standpoint of controlling the flow of heat in the desired manner.
{tocify} $title={Table of Contents}
It is a well-established fact that if two bodies at different temperatures are brought into thermal contact, heat flows from a body at a high temperature to that at a lower temperature (Second law of thermodynamics). The net flow of heat is always in the direction of temperature decrease. Thus, heat is defined as a form of energy that is in transit between a hot source and a cold receiver.
The transfer of heat solely depends upon the temperatures of the two bodies/substances/parts of the system, i.e., the high temperature of a body is the indication of a high level of the heat energy content of the body.
Basic Mechanisms of heat transfer
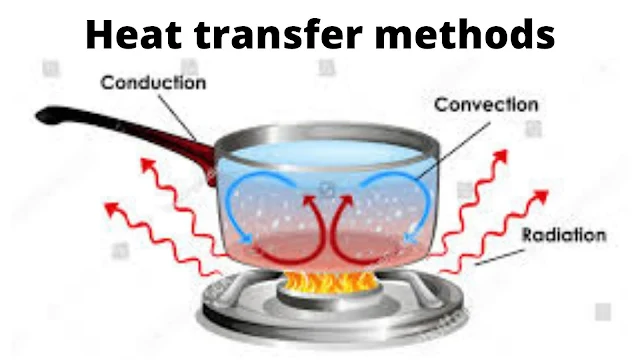
Whenever the temperature difference (driving force for heat transfer) exists between two parts of a system, the heat may flow by one or more of the three basic mechanisms, namely, conduction, convection, and radiation. We will first see three modes of heat transfer in brief and then we will consider heat conduction through solids in detail.
Heat Transfer By
Conduction heat transfer
It is the transfer of heat from one part of a body to another part of the same body or from one body to another which is in physical contact with it, without appreciable displacement of particles of a body. In metallic solids, thermal conduction results from the motion of unbound electrons. It is restricted to the flow of heat in solids.
Conduction Examples
Example of conduction: Heat flow through the brick wall of a furnace, the metal sheet of a boiler, and the metal wall of a heat exchange tube.
Convection heat transfer
It is the transfer of heat from one point to another point within a fluid (gas or liquid) by mixing hot and cold portions of the fluid. It is attributed to the macroscopic motion of the fluid. Convection is restricted to the flow of heat in fluids and is closely associated with fluid mechanics. In natural convection, the motion of the fluid is produced by mechanical means such as an agitator, a fan, or a pump.
Examples of heat transfer mainly by convection are heating of a room using a steam radiator, heating of water in cooking pans, and heat flow to a fluid pumped through a heated pipe.
Radiation heat transfer
Radiation refers to the transfer of heat energy from one body to another, not in contact with it, by electromagnetic waves through space.
Examples of heat transfer by radiation: Heat transfer by radiation mode is the transfer of heat from the sun to the earth and loss of heat from an unlagged steam pipe.
Conduction can occur in solids, liquids, and gases while convection occurs only in the presence of a material medium whereas radiation can occur even in a vacuum and no material medium is required for heat flow by radiation. It is observed that heat flow by conduction is slow, faster by convection, and the fastest by radiation mode.
Conduction heat transfer
It is our common observation that when some material object is heated at one of its locations then in a short while its remaining parts are also heated. This shows that heat flows through the material object from a high-temperature region to a low-temperature region. The flow of heat in this manner is called heat conduction or simply conduction wherein the particles of the object participate in the process but they do not move bodily from the hot or high-temperature region to the cold or low-temperature region.
Conduction is the mode of heat transfer in which a material medium transporting the heat remains at rest. Heat conduction occurs by the migration of molecules and more effectively by the collision of the molecules vibrating around relatively fixed positions. In liquid and solids where little or no migration occurs, heat is transferred by the collision of vibrating molecules.
Conduction refers to the mode of heat transfer in which the heat flow through the material medium occurs without the actual migration of particles of the medium from a region of higher temperature to a region of lower temperature.
It is a fact that conduction occurs in solids, liquids,s, and gases but pure conduction is found to present only in solids, with gases and liquids it is present with convention, so we will consider here heat conduction in solids for a better understanding of conduction mechanism as a convention is not present in solids.
Take these Notes is, Orginal Sources: Unit Operations-II, KA Gavhane