In distillation as in the distilllation of binary mixture, the calculation of equilibrium stages uses mass and enthalpy balances and vapour-liquid equilibria.
A mass balance can be written for each component for the column or for each stage. When separation by distillation is ineffective or very difficult, liquid extraction is one of the main alternatives to consider.
The close-boiling mixture of substances that cannot withstand the temperature of distillation even under a vacuum may often be separated from impurities by extraction, which utilizes chemical differences instead of vapour pressure differences.
{tocify} $title={Table of Contents}
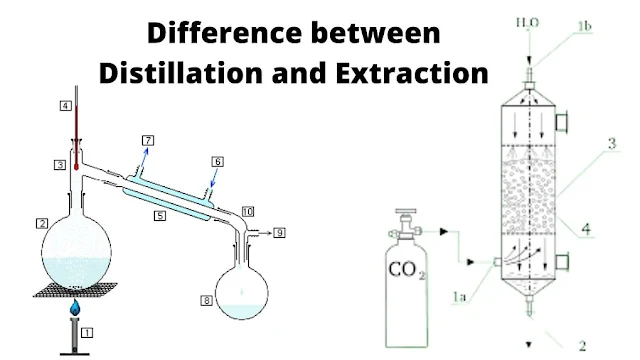
Difference Between Distillation and Extraction
Distillation process
1. Distillation/fractionation
is an operation in which the constituents of a liquid mixture are separated by
using thermal energy.
2. Distillation
utilizes differences in vapour pressure of different components at the same
temperature to affect a separation.
3. In
distillation, relative volatility is used as a measure of the degree of
separation.
4. In
distillation, a new phase is created by the addition of heat.
5. In
distillation mixing and separation of phases is easy and rapid
6. Distillation/fractionation
gives almost pure products.
7. It does not
offer more flexibility in the choice of operating conditions.
8. It requires
thermal energy.
9. It needs heating
and cooling provisions.
10. It is a
primary choice for the separation of the components of a liquid mixture.
11. Extractive
distillation is the extraction of the vapour phase with solvent.
Extraction Process
1. Extraction
is an operation in which the constituents of a liquid mixture are separated by
using an insoluble liquid solvent.
2. Extraction utilizes
differences in the solubility of components to effect a separation.
3. In extraction,
selectivity is used as a measure of the degree of separation.
4. In
extraction, a new insoluble liquid phase is created by the addition of solvent
to the original mixture.
5. In
extraction, phases are hard to mix and harder to separate.
6. The
extraction itself does not give pure products and needs further processing.
7. It requires
mechanical energy (for mixing and separation).
8. It does not
need heating and cooling provisions.
9. It is a secondary
choice for the separation of the components of a liquid mixture.
10. Liquid
extraction is the extraction of the liquid phase with solvent.
Take these Notes is, Orginal Sources: Unit Operations-II, KA Gavhane