The Langmuir adsorption isotherm and equation derivation, considers gas molecules striking a solid surface some of these molecules then evaporate or adsorb a dynamic equilibrium is established blow the two opposing processes adsorption and desorption.
Langmuir Adsorption Isotherm Derivation
The θ is the total surface covered by adsorbed naked area (1- θ ). The rate of desorption is directly proportional to the covered surface θ.
Rd ∝ θ
Rd = K1θ
K1 is a constant wish that represents the rate at which molecules evaporate from the covered surface. The rate of adsorption is directly proportional to the naked surface (1 - θ ) and the pressure of a gas
Ra ∝ (1 - θ)
Ra = K2 (1 - θ)
K2 is proportionally constant
At equilibrium rate of adsorption becomes equal to the rate of desorption.
K1 θ = K2(1 - θ)P
K1 θ = K2P - K2θP
K1θ+K2θp = K2P
θ = K2P / K1+K2P
θ = K2/K1P / K1/K1 + K2/K1 -------------------(1)
Where b = K2/K1 and is called the adsorption coefficient. So equation (1) becomes
θ = bP/1+bP ----------------------(2)
Equation (2) is Langmuir isotherm we can also write it.
The amount of gas adsorption per unit area or per unit mass of the adsorbent must be proportional to the fraction of the surface covered.
y = x/m = K θ --------------(3)
Put equation (2) in equation (3)
y = x/m =K. bP/1+bP
Where constant a = Kb
y = x/m = aP/1+bP ------------(4)
Constant a and b are characteristics of the system under consideration and are evaluated from experimental data. Equation number (4) is the actual Langmuir adsorption isotherm. value of a and b depend upon the nature of the gas adsorption, the nature of the adsorbent, and temperature.
Equation (4) can also be written.
x/m/p = aP/P(1+bP)
x/m/P = a/(1+bP) OR
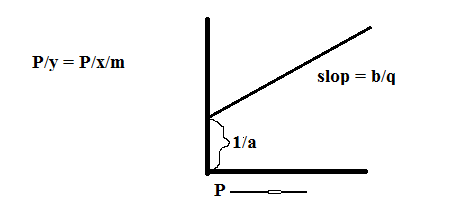
P/x/m = 1+bP/a
P/x/m = 1/a+b/aP
y = C+mx
Use of Langmuir isotherm and Limitations
(i) Langmuir adsorption isotherm gives a quantitative explanation of adsorption.
(ii) This equation explains the mechanism of chemisorption.
(iii) It is more satisfactory than Freundlich's theory while explaining the physical adsorption of gases on solids.
(i) This theory could not explain all the experimentally observed adsorption isotherms.
(ii) This theory holds good at low pressure only.
(iii) This theory assumes that the surface is capable of adsorbing a layer one molecule thick and no more but in actual practice, much thicker layers have been reported.
(vi) According to this theory the saturation value of adsorption is independent of temperature but experiments show that saturation value decreases with rising in temperature.
>Determination of oil adsorption.
>Organization Structure for TPM Implementation and Pillars of TPM